ADVERTORIAL
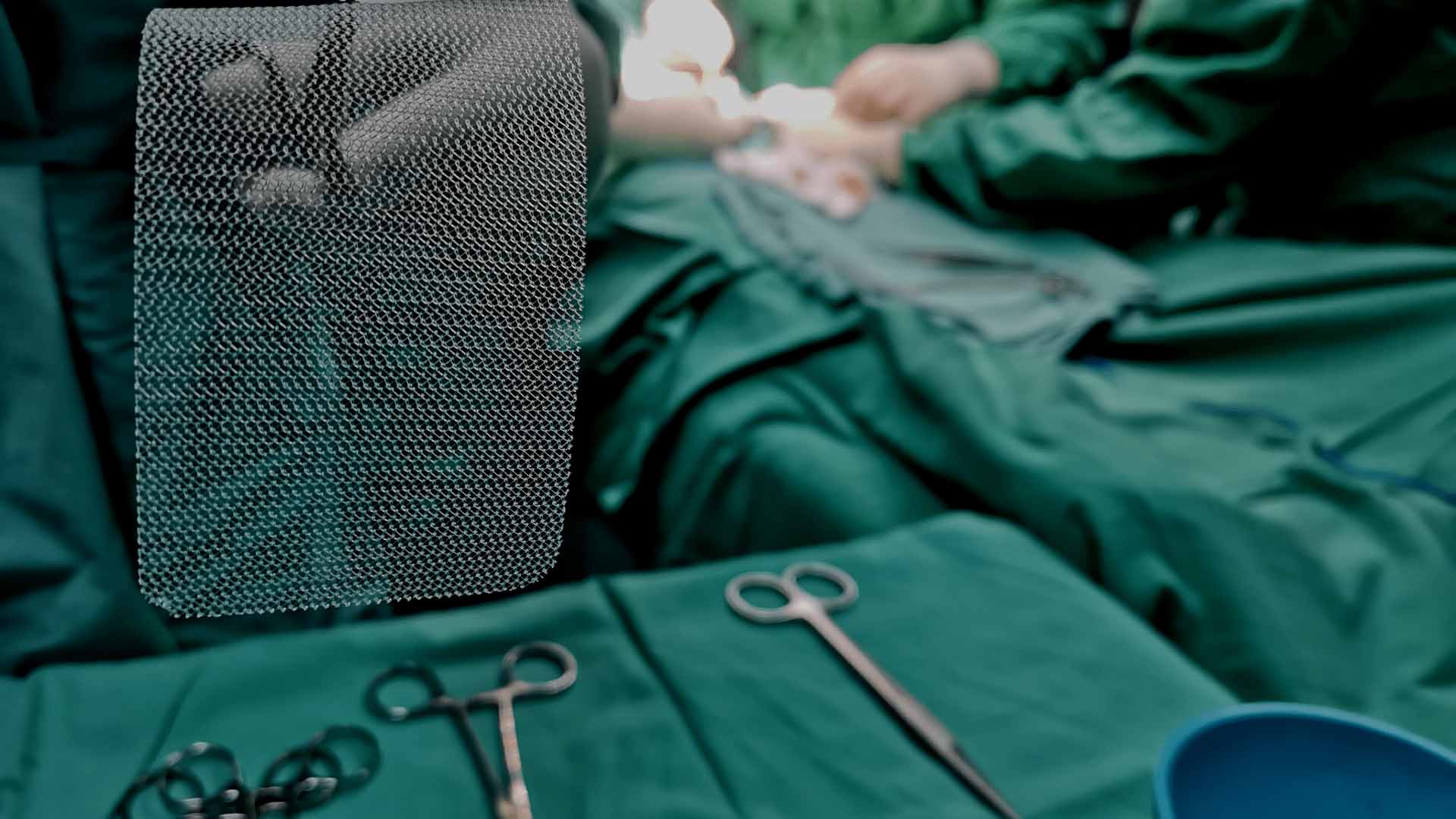
Hernia mesh is used to support damaged tissue after hernia surgery to give the patient more mobility and expedite their recovery. Unfortunately, some patients that undergo hernia repair surgery suffer severe complications, often due to the implanted hernia mesh.
There have been numerous hernia mesh recalls over the past 15 years by both the FDA and the manufacturers. Some types of hernia mesh, like Physlomesh and C Qur mesh, have been linked to damage of the intestines, bowels, and abdomen. These side effects often require additional surgery.
A 2016 study with 3,242 participants who had hernia mesh surgery found that the number of complications increased over the course of five years. 1,050 individuals in the study required additional abdominal surgery to correct these complications later on.
The most serious hernia mesh complications are often deadly.
Manufacturers of hernia mesh such as Boston Scientific, C.R. Bard, and Ethicon have known about the severe implications of this surgery, yet have FAILED TO WARN CONSUMERS ABOUT THESE RISKS.
Adverse event reports from health care professionals to U.S. Food and Drug Administration grew. Eventually, and arguably belatedly, the FDA issued safety warnings and demand product recalls.
The Dangers of Polypropylene Hernia Mesh
While polypropylene has been used in various medical procedures for decades, the advancement of using polypropylene in more areas of surgical repair has not been without significant controversey. That is especially true regarding the use of polypropylene mesh in the repair of stress urinary incontinence, pelvic organ prolapse, and hernia repairs.
Polypropylene is a petroleum by-product used to manufacture a variety of items such as fishing line. Polypropylene can cause an immunological response when implanted in the human body which can lead to serious infections. Polypropylene can also degrade in the human body.
Hernia Mesh Complications
Some patients have alleged that hernia mesh complications caused them injuries to their abdomen, bowels, and intestines. May times requiring additional, corrective surgeries. Some of the most common hernia mesh complications and injuries include:
- Pain
- Mesh migration or movement
- Shrinkage
- Hernia recurrence
- Revision surgeries
- Infections
- Mesh adhesion
- Bowel perforation and restriction
To better illustrate the gravity of the situation, below is a list of surgical mesh lawsuit settlements and trial awards resulting from surgical mesh implants used in pelvic and hernia mesh repair operations:
- Johnson & Johnson - $154 Million
- CR Bard - $319 Million
- Boston Scientific - $34.5 Million
- Endo International - More than $1 Billion
- Boston Scientific - $45.2 Million
- CR Bard - $184 Million
Thousands of hernia mesh lawsuits are currently pending against different medical device manufacturers. However, most estimates suggest that there are still thousands of potential hernia mesh plaintiffs that have not yet filed. Although none of the current round of hernia mesh device lawsuits have settled or gone to trial yet, prior verdicts and settlements from comparable cases suggest that hernia mesh claims will likely be worth $500,000 to $1,000,000 depending on individual circumstances.
If you received a hernia mesh product and underwent additional surgeries to remove the mesh or repair damage caused by the mesh, you now have the opportunity to fight back. By filing a hernia mesh lawsuit, you could obtain a settlement or judgement to recover your financial losses and compensate you for pain and suffering.
What Do You Need To Do To Claim It?
Step 1: Click the red button below.
Step 2: On the next page, answer a few questions about your personal situation (takes 30 seconds).